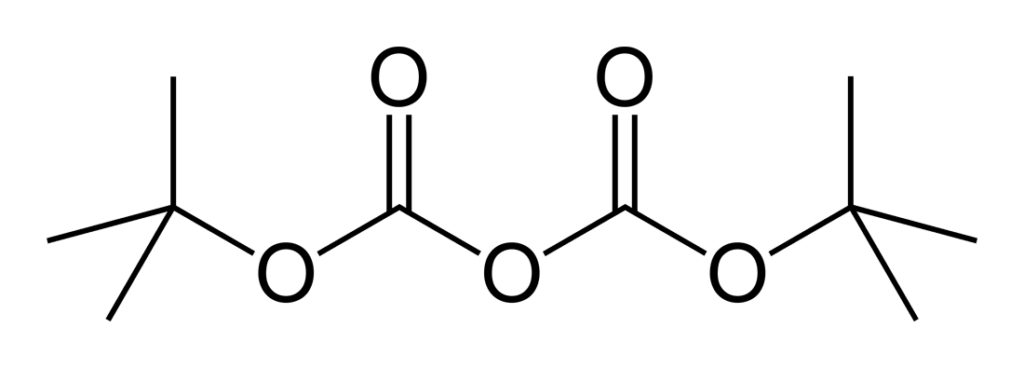
Specifications of Di-tert-butyl dicarbonate (DIBOC)
Appearance: Colorless liquid/solid
Gardner Color: ≤0.2 gardner color
Particulate: Free of particulates
Identity (FT-IR): Conforms to standard
Purity Assay (GC): ≥99.0%
Properties of Di-tert-butyl dicarbonate (DIBOC)
The Boc group is used extensively in amino acid, peptide and heterocyclic synthesis for amine protection.1 It is not readily hydrolyzed under basic conditions and is inert to many nucleophilic reagents. It is usually cleaved with strong acids, giving only t-BuOH or isobutylene and CO2 as by-products. As a result, it is one of the most commonly used protective groups for amines.
In general, the Boc group is considered nonreactive, but there are many cases in which the BOC group participates in reactions.2 An example of note is when an internal nucleophile can close to give an oxazolidinone ring.
In the presence of a nucleophilic catalyst such as DMAP, a simple aliphatic amine can be doubly Boc protected. With aromatic amines, treatment with DiBoc in the presence of DMAP can be used as an effective (phosgene free) means of preparing isocyanates.